To overcome the limitations of current screening methods for prostate cancer¹ ², Philips has developed UroNav*, an image-guided stereotactic biopsy system that has the ability to improve the sensitivity and specificity of prostate biopsies. It has the potential to reduce false negatives and speed up diagnosis, which can make a life-saving difference to patients.
* This product is not for the distribution in the USA
The need for targeted prostate biopsies
Current methods of prostate cancer screening, such as prostate-specific antigen (PSA) tests and digital rectal exams (DRE), are somewhat unreliable and can lead to many uncertainties for both patient and urologist. Prostate biopsy, the most reliable method of detection, is a challenge because of the difficulties in visualizing not only the entirety of the prostate, but also the location of the biopsy needle. Trans-rectal ultrasound-guided prostate biopsy (TRUS), the current biopsy standard, commonly suffers from poor image resolution, and the biopsy needle often passes through tumor-free areas of the prostate – potentially missing the tumor entirely.
UroNav – image-guided prostate biopsies
UroNav is an image-guided stereotactic biopsy system used to detect prostate cancer within patients as an alternative to current blind or blind systematic biopsies. It uses multi- parametric magnetic resonance (mpMR) imaging, fused with live ultrasound (US) guidance in conjunction with electromagnetic (EM) tracking to plan, guide, and document prostate biopsies. UroNav simultaneously displays registered MR and ultrasound images and the projected needle path relative to the suspicious target lesion during the biopsy procedure and guides the urologist in real-time.
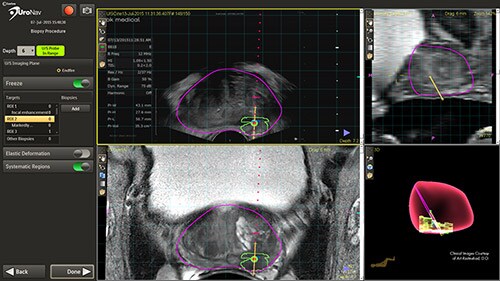
Earlier diagnosis –the pathway to better outcomes
This novel approach has the ability to improve the sensitivity and specificity of prostate biopsies. This results in a reduced incidence rate of false negative biopsy results. Conventional biopsy or “blind” procedures have a far lower cancer detection rate, which affects patient outcomes by delaying diagnosis, treatment intervention, and limiting treatment options. By enabling earlier diagnosis, UroNav allows a broader array of treatment options to be considered by the clinician, less complications for the patient, and a more cost-effective resolution for the care provider. From set up to post-biopsy review, UroNav guides you through its intuitive workflow. After a quick initialization of the navigation system (upper image), UroNav creates a 3D ultrasound volume from a standard 2D scan of the prostate (lower image).
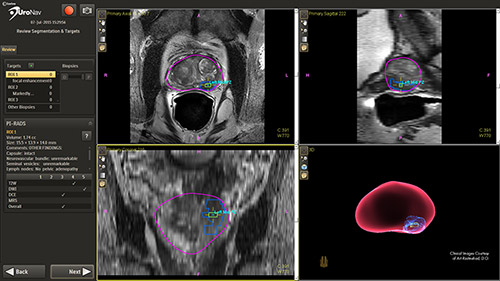
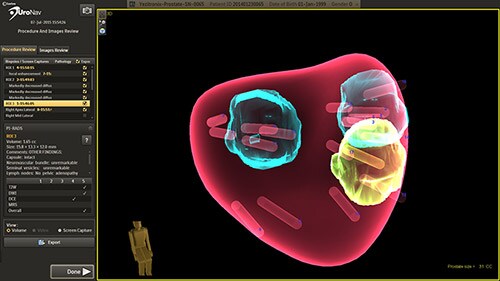
Clinical studies
Clinical studies are being carried out by clinical partners, including Dr. P. Pinto at the National Institutes of Health (NIH), Washington, DC and A.R. Rastinehad at The Arthur Smith Institute for Urology, Hofstra North Shoreu2010LIJ School of Medicine, New Hyde Park, NY.
¹ Pinsky PF, Black A, Parnes HL. Prostate cancer specific survival in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Cancer Epidemiol. 2012 Dec;36(6):e401‐6. ² Schröder FH, Hugosson J, Roobol MJ. Screening and prostate‐cancer mortality in a randomized European study. N Engl J Med. 2009 Mar 26;360(13):1320‐8. Images courtesy of Long Island Jewish Medical Center, USA
This product is not for distribution in the USA


